Electricity

You can describe electricity through units of volt and ampere - each measure two different things. A volt measures the size of the force that sends the electrons through a circuit. An ampere measures electrical current, counting the number of electrons flowing through a circuit.
If you think of electrons as driftwood floating in a river, volt measures the water‘s speed, ampere measures the amount of driftwood in the water.
What all electric circuits share is that they entail an anode and a cathode, a plus and a minus pole. Electrons flow from cathode (minus) to anode (plus), while current goes from anode (plus) to cathode (minus).
Electrolyte
Electrolyte is a chemical substance that, when dissolved in water, dissociates into electrically charged particles (ions) and is capable of conducting an electric current. In our bodily fluids, there are principal positively charged ions (cations), and negatively charged ions (anions). Fruit and vegetable juices can also dissociate into electrically charged particles and can conduct an electric current. This means you can use different juices as electrolytes.
DIY batteries

Take a lemon for example. By pushing a piece of zinc metal (like a galvanized nail) and a piece of copper (like a copper coin) into your lemon, you create electrodes. They make electrically charged particles (ions) move from the more valuable metal to the less valuable metal (from copper to zinc) with a by-product of hydrogen. This is due to a chemical process called reduction. This experiment creates a very simple battery.
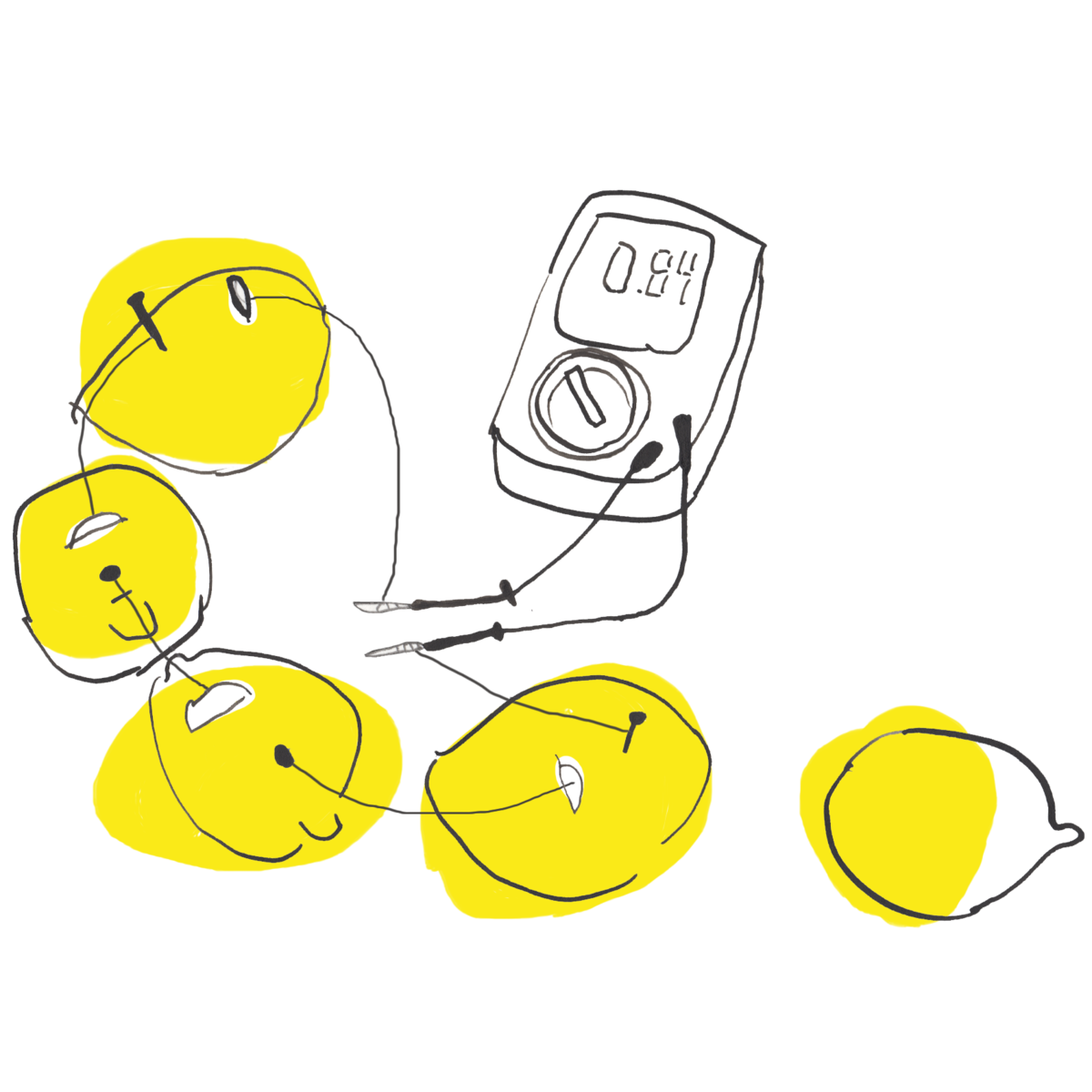
The first battery of this kind was invented by Alessandro Volta, around 1800. Once you have attached the zinc metal and copper coin to your lemon you can measure the volt that this battery generates through a measuring tool, called a [multimeter](####multimeter). You can try to connect the shorter leg of an LED with the zinc metal and the longer leg of an LED with the copper and see if the LED lights up.
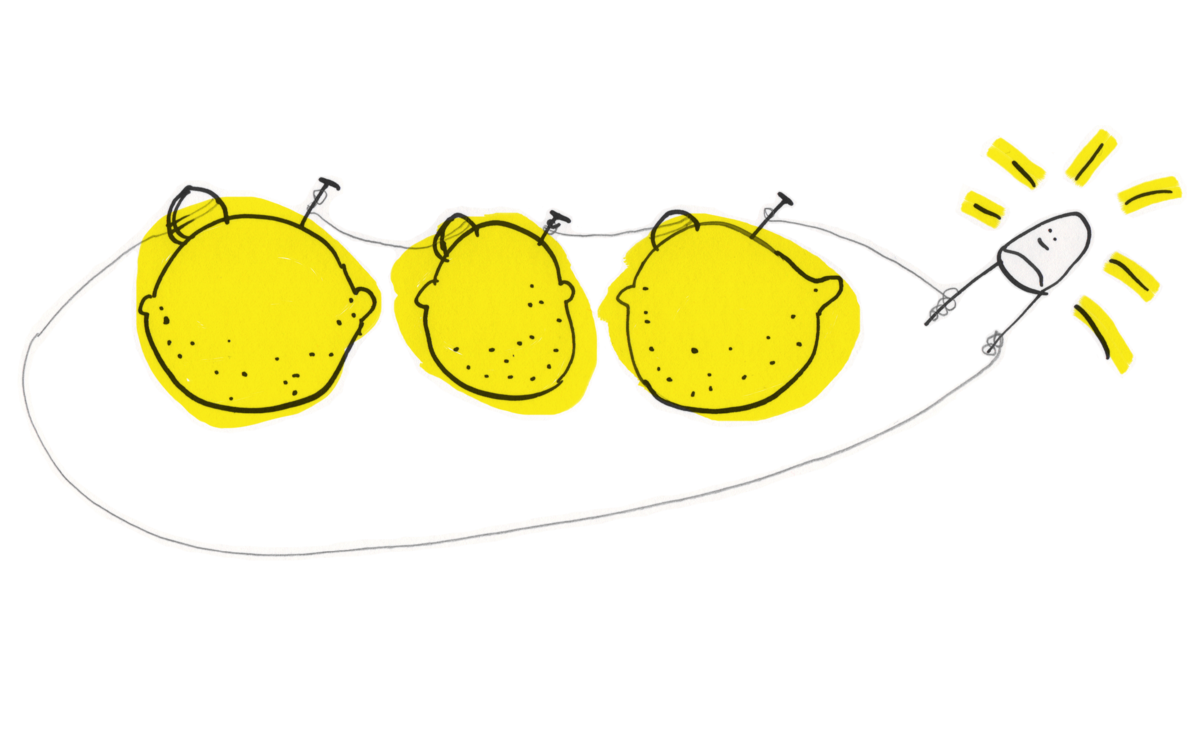
If you substitute zinc metal and copper with magnesium and copper, it will make the lemon battery produce a higher voltage.
You can also play with the order of multiple lemon batteries: you can connect their wires in parallel (all zinc wires and all copper wires connected) and they will give you different output than when connected in serial (copper wires connected to the zinc wires of the next lemon which again is connected to the copper of the next and so forth).
This experiment can be done with all kinds of organic materials, like Sauerkraut, potatoes and even trees (e.g. the Ambarella tree). Just make sure you don‘t eat any of the things you used as a battery.
TOOLS
Multimeter
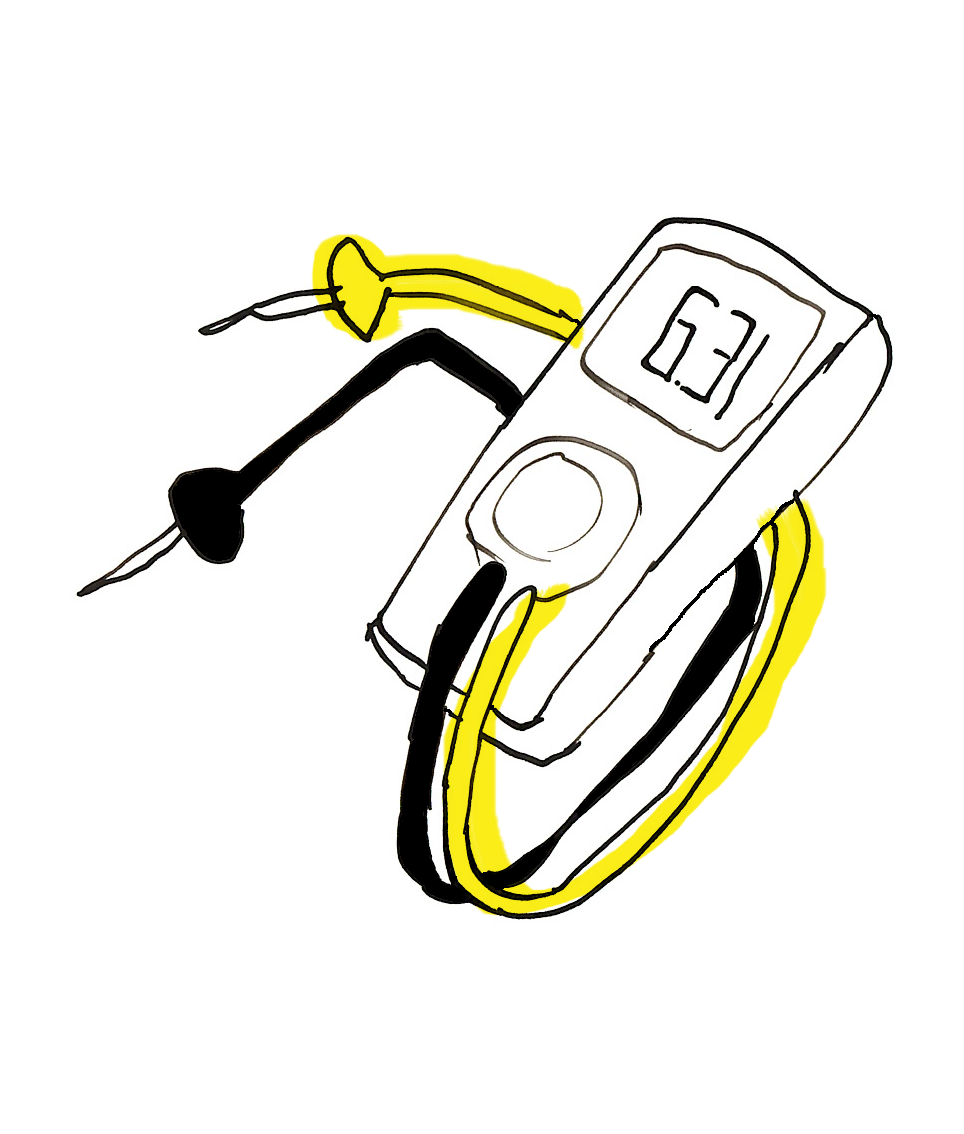
The multimeter helps to measure volt (electric potential), ampere (electric current) and ohm (resistance). If you would like to measure how much volt a battery is charged with, the end of the multimeter black wire needs to touch the cathode and the end of the red multimeter wire needs to touch the anode of the battery. The little wheel enables you to measure signals in different ranges.
Alligator clips

Alligator clips serve as wires that can be attached and detached from a circuit board quickly, in order to make fast prototype circuits. The clips have little teeth like alligators. It‘s good to buy a couple of them in different colors.
LED

A light-emitting diode (LED) is a little lamp with two wires, one the anode, one the cathode. If you connect the shorter wire (cathode) to the minus pole of a battery and the longer wire (anode) to the plus pole of a battery it will light up.